Levi & Korsinsky Announce AVEO Lawsuit; AVEO Class Action
Levi & Korsinsky, LLP
March 15, 2019
Hackel v. Aveo Pharmaceuticals, Inc. et al 1:19-cv-01722-AT — On February 25, 2019, investors sued AVEO Pharmaceuticals, Inc., (“AVEO” or the “Company”) in United States District Court, Southern District of New York. The AVEO class action alleges that plaintiffs acquired AVEO stock at artificially inflated prices between August 4, 2016 and January 31, 2019 (the “Class Period”). They are now seeking compensation for financial losses incurred upon public revelation of the Company’s alleged misconduct during that time. For more information on the AVEO lawsuit, please contact us today.
Summary of the Allegations
Company Background
The Company (NASDAQ: AVEO) is a biopharmaceutical company known as GenPath Pharmaceuticals, Inc., until it adopted its current name in 2005. As such, it says it is “dedicated to advancing a broad portfolio of targeted medicines for oncology and other areas of unmet medical need.”
To that end, AVEO has adopted a strategy in which it retains the rights to its “oncology portfolio” in North America while it engages in partnerships for the development and commercialization of its products elsewhere.
The Company’s lead product candidate is tivozanib or FOTIVDA®, which was created to treat advanced or metastatic renal cell carcinoma (“RCC”). AVEO claims that it is approved in the European Union, as well as Norway and Iceland, for the first-line treatment of adult patients with RCC and related conditions.
AVEO’s statements about the U.S. Food and Drug Administration (FDA) approval of tivozanib and related issues are at the crux of the February 25 complaint.
Summary of Facts
AVEO and three of its current and/or former senior officers (the “Individual Defendants”) are now accused of deceiving investors by lying and withholding critical information about the Company’s business practices, operational and compliance policies during the Class Period.
Specifically, they are accused of omitting truthful information about the viability of AVEO’s lead product candidate from SEC filings and related material. By knowingly or recklessly doing so, they allegedly caused the Company’s stock to trade at artificially inflated prices during the time in question.
The truth came out in a Boston Business Journal article published on January 31, 2019. In it, the Journal reported that, “AVEO would not submit its tivozanib application for FDA approval ‘due to a recommendation from the agency [to] gather more late-stage testing results. Specifically, the FDA is asking for additional survival data, echoing concerns that led to the agency’s rejection of the same drug in 2013.’”
A closer look…
As alleged in the February 25 complaint, the Company and/or Individual Defendants repeatedly made false and misleading public statements during the Class Period.
For instance, on a form filed with the SEC at the outset of the Class Period, the Company stated in relevant part: “In May 2016, we initiated enrollment and treatment of patients in our new phase 3 trial of tivozanib in the third-line treatment of patients with refractory RCC. The TIVO-3 trial was designed to address the OS concerns from the TIVO-1 trial presented in the June 2013 complete response letter from the FDA and to support a request for the regulatory approval of tivozanib in the United States…”
On another form filed with the SEC on March 22, 2017, AVEO also stated in relevant part: “The TIVO-3 trial passed an initial safety data assessment in February 2017.”
Finally, on yet another form filed with the SEC on March 13, 2018, the Company stated in pertinent part: “The primary objective of the TIVO-3 trial is to show improved PFS. Secondary endpoints include OS, safety and ORR.”
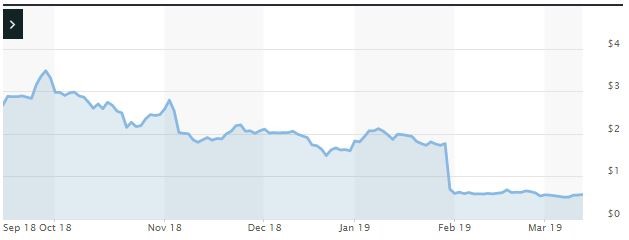
Impact of the Alleged Fraud on AVEO’s Stock Price and Market Capitalization
| Closing stock price prior to disclosures:
|
$1.77 |
| Closing stock price the trading day after disclosures:
|
$0.70 |
| One day stock price decrease (percentage) as a result of disclosures:
|
60.45% |
The following chart illustrates the stock price during the class period:
Actions You May Take
If you have purchased shares during the Class Period, you may join the class action as a lead plaintiff, remain a passive class member, or opt out of this litigation and pursue individual claims that may not be available to the class as a whole.
NOTE: The deadline to file for lead plaintiff in this class action is April 26, 2019. You must file an application to be appointed lead plaintiff prior to this deadline in order to be considered by the Court. Typically, the plaintiff or plaintiffs with the largest losses are appointed lead plaintiff.
In order to identify your potential exposure to the alleged fraud during the time in question, you may wish to perform an analysis of your transactions in AVEO common stock using court approved loss calculation methods.
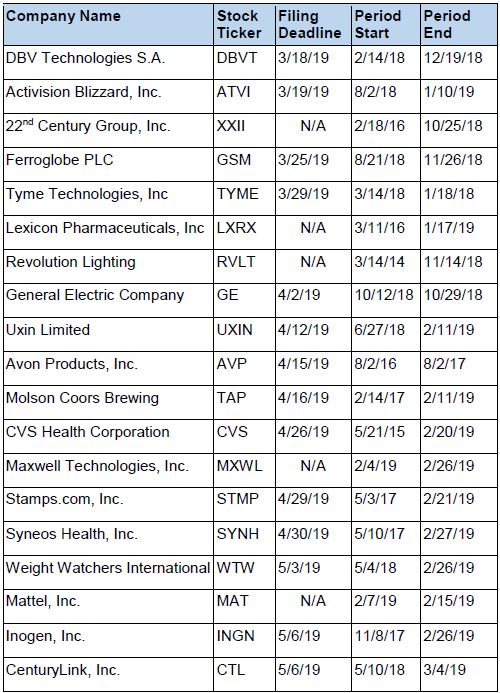
Recently Filed Cases
Listed below are recently filed securities class action cases being monitored by us, along with the class period and the deadline to file a motion to be appointed as the Lead Plaintiff in the action. Please contact us if you would like an LK report for any of these cases:
About Us
Levi & Korsinsky is a leading securities litigation firm with a hard-earned reputation for protecting investors’ rights and recovering losses arising from fraud, mismanagement and corporate abuse. With thirty attorneys and offices in New York, Connecticut, California and Washington D.C., the firm is able to litigate cases in various jurisdictions in the U.S., England, and in other international jurisdictions.
Levi & Korsinsky provides portfolio monitoring services for high-net worth investors and institutional clients. Our firm also assists investors in evaluating whether to opt-out of large securities class actions to pursue individual claims.
For additional information about this case or our institutional services, please contact us.